Introduction
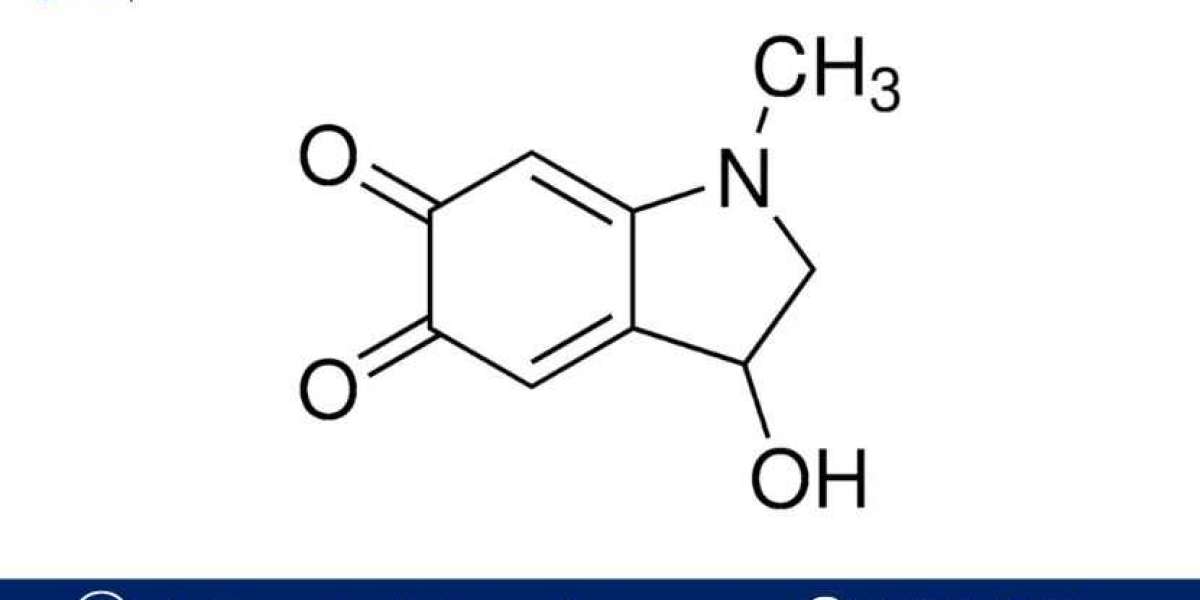
An Adrenochrome Manufacturing Plant Project Report provides a detailed guide for establishing a production facility focused on the manufacturing of adrenochrome, a compound derived from the oxidation of adrenaline (epinephrine). Adrenochrome has gained notoriety in popular culture and conspiracy theories, though it is of interest in scientific and medical research due to its potential effects on the human body. The compound has been studied for its possible connection to schizophrenia and other neurological conditions, although these studies have been limited.
This project report will offer essential details for setting up a plant that can produce adrenochrome, covering the necessary equipment, raw materials, processes, regulatory considerations, financial projections, and market opportunities for businesses interested in entering the field. It aims to provide entrepreneurs and industry stakeholders with a step-by-step approach for entering the niche market of adrenochrome production.
Market Overview and Industry Analysis
Adrenochrome is a naturally occurring substance produced through the oxidation of adrenaline, which is itself a hormone released during stress and the "fight or flight" response. While the compound has garnered interest from both the medical community and various alternative health circles, its commercial production is limited, and there is little mainstream demand for it as a consumer product.
Medical and Scientific Applications
Adrenochrome has been researched primarily for its potential use in the treatment of schizophrenia and other psychiatric disorders, although clinical evidence supporting its therapeutic efficacy is scarce. The compound is also studied for its possible role in the development of neurodegenerative diseases, as it has been suggested that it may have antioxidant properties.
In addition to potential medical applications, adrenochrome has been explored in the field of cosmetic products, though its usage remains niche. Many of the purported benefits, particularly those related to anti-aging and cognitive enhancement, are still under investigation and have not been conclusively proven in scientific trials.
Get a Free Sample Report with Table of Contents@
Limited Commercial Demand
Currently, adrenochrome is not a widely produced or sold compound. The primary industries involved in its production and distribution are those engaged in pharmaceuticals, research, and potentially alternative medicine. Its production is regulated in many countries due to its potential implications for human health and its association with certain medical conditions.
However, there may be growing interest in adrenochrome due to the increasing focus on personalized medicine and the search for novel compounds with psychoactive or therapeutic properties. This opens up a potential market niche for businesses willing to invest in research, development, and production.
Key Components of the Project Report
The Adrenochrome Manufacturing Plant Project Report should address several critical aspects of establishing a production facility, including market analysis, plant design, production processes, raw materials, machinery requirements, regulatory compliance, and financial projections. Below is an outline of the key components of such a report.
1. Executive Summary
The executive summary provides an overview of the adrenochrome manufacturing project, including:
- Business Objectives: The goals of the manufacturing plant, such as production capacity, target markets, and revenue expectations.
- Product Focus: Adrenochrome, produced for research or niche medical applications.
- Financial Overview: The expected initial investment, projected revenues, and profitability.
- Market Opportunity: A brief overview of the demand for adrenochrome in medical research, pharmaceuticals, and alternative medicine.
2. Business Model and Objectives
The business model for an adrenochrome manufacturing plant should focus on the following:
- Product Range: The primary product will be adrenochrome, with potential variations depending on the form in which it is produced (e.g., crystalline powder, liquid extract).
- Target Market: The key markets for adrenochrome include pharmaceutical companies, research institutions, and alternative medicine sectors. Some potential applications include research into neurological disorders and psychiatric conditions.
- Revenue Streams: Revenue may come from direct sales to pharmaceutical companies, research institutions, or private medical companies. There may also be opportunities to license the technology to other manufacturers or research entities.
- Sales and Marketing Strategy: Develop a comprehensive marketing and sales strategy, emphasizing the scientific and medical benefits of adrenochrome. Consider strategic partnerships with universities, research labs, or pharmaceutical companies for continued research and product development.
3. Plant Design and Machinery Requirements
Plant Layout
The plant layout is crucial for ensuring efficient production and maintaining high standards of hygiene and safety. Essential areas of the plant include:
- Raw Material Storage: An area for storing the raw materials required to produce adrenochrome, including adrenaline and other chemicals used in the oxidation process.
- Production Area: The main area where the adrenochrome is synthesized through chemical reactions. This area will be equipped with the necessary chemical reaction vessels and equipment.
- Quality Control and Testing: A dedicated section for testing the quality and purity of the produced adrenochrome. This includes chemical analysis, stability testing, and safety testing.
- Packaging Area: After quality testing, the adrenochrome will be packaged for distribution.
- Storage and Distribution: Proper storage conditions for finished products, especially if refrigeration is required, and logistics for shipping the product to customers.
Machinery and Equipment
The machinery and equipment required for adrenochrome manufacturing include:
- Chemical Reaction Vessels: These are required to carry out the oxidation of adrenaline to form adrenochrome under controlled conditions.
- Purification Equipment: Various filtration and purification systems are necessary to ensure the adrenochrome produced is of high purity.
- Analytical Instruments: To measure the concentration, purity, and stability of the compound. These could include spectrometers, chromatography systems, and mass spectrometers.
- Drying Equipment: If the adrenochrome is produced in a powdered form, drying systems will be required to convert it from liquid to powder.
- Packaging Machines: To package the final product in bottles, vials, or other containers.
- Storage and Refrigeration: For maintaining the stability of the final product, especially if it needs to be stored under controlled temperatures.
4. Raw Materials and Suppliers
The key raw material for adrenochrome production is adrenaline (epinephrine), which is typically sourced from pharmaceutical-grade suppliers. Other raw materials include:
- Oxidizing Agents: Chemicals required for the oxidation process to convert adrenaline into adrenochrome.
- Solvents and Reagents: Used during the synthesis and purification stages to aid in the chemical reactions and extract the final product.
- Packaging Materials: Bottles, vials, and sealing equipment for the finished product.
Establishing reliable relationships with raw material suppliers is essential to maintain consistent production and meet regulatory standards.
5. Production Process
The production process for adrenochrome involves the following key steps:
- Adrenaline Extraction: Adrenaline is extracted from natural sources (e.g., animal glands) or synthesized in the lab.
- Oxidation: The adrenaline undergoes a controlled oxidation process using an oxidizing agent to form adrenochrome.
- Purification: The crude adrenochrome is purified through a series of chemical reactions, filtration, and chromatography techniques.
- Testing and Analysis: The purity and concentration of the adrenochrome are tested using sophisticated analytical equipment to ensure it meets regulatory standards.
- Packaging: The purified adrenochrome is packaged for sale or distribution.
6. Regulatory Compliance
Adrenochrome production is subject to several regulatory requirements, including:
- Good Manufacturing Practices (GMP): Compliance with GMP is essential for ensuring the safety and quality of the product.
- FDA Regulations: If the product is intended for use in pharmaceuticals or medical treatments, it must comply with the Food and Drug Administration (FDA) regulations in the U.S. or corresponding agencies in other countries.
- Environmental Regulations: Compliance with environmental laws regarding the disposal of chemical waste and the use of hazardous substances.
- Safety Standards: Adhering to safety protocols for handling chemicals and ensuring worker safety during the production process.
7. Financial Projections
Financial projections for the adrenochrome manufacturing plant should include:
- Initial Investment: The cost of setting up the plant, including equipment, facilities, raw materials, and labor.
- Operating Costs: Ongoing costs for raw materials, labor, energy, and maintenance.
- Revenue Projections: Estimate the revenue based on the expected production capacity, pricing strategy, and market demand.
- Profitability: Calculate the breakeven point and expected return on investment (ROI).
- Contingency Plans: Include provisions for unforeseen expenses and challenges in the manufacturing process.
8. Marketing and Distribution Strategy
Given the niche nature of the adrenochrome market, marketing and distribution strategies will need to focus on establishing a strong network with pharmaceutical companies, research institutions, and alternative medicine providers. Key strategies include:
- B2B Sales: Establishing direct partnerships with pharmaceutical manufacturers, research facilities, and medical institutions.
- Licensing: Licensing the production technology to other manufacturers or research entities.
- Online Marketing: Engaging in online marketing to promote adrenochrome's potential applications in research and healthcare.
9. Risk Analysis and Mitigation
Several risks should be considered:
- Regulatory Challenges: Changes in regulations or new restrictions could impact production and distribution. Ongoing compliance is critical.
- Market Demand: Adrenochrome is a niche product, and its market demand could fluctuate. Diversifying the product range or exploring related markets may help mitigate this risk.
- Supply Chain Disruptions: Delays or shortages in raw materials could impact production schedules. Establishing reliable supplier relationships and maintaining a buffer stock can reduce this risk.
Explore More Reports
https://www.expertmarketresearch.com/articles/top-washing-machine-companies
Media Contact
Company Name: Claight Corporation
Contact Person: Peter Fernandas, Corporate Sales Specialist — U.S.A.
Email: [email protected]
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au